
Bradford's collaboration boosts surprising discovery of seeds’ nutrition ‘gate’
Chance encounter with Japanese peer plants a seed for larger grains
A recent discovery getting attention in scientific circles points toward a new pathway for growing larger grains, beans and other edible seeds. In addition, the research -- which is getting a boost from Kent Bradford in the UC Davis Department of Plant Sciences – shows the impact of the department’s international collaborations.

A team of researchers in Japan has shown how callose – a structural molecule involved in many basic activities of plant cells – plays a key role in controlling the delivery of nutrients to developing seeds. By manipulating the gene that controls a callose “gateway” into the seed, the scientists produced seeds that were significantly larger, both in thale cress -- a model plant -- and in rice, one of the world’s most important food crops. Bradford is one of the authors named in a paper describing the breakthrough, which was published recently in Current Biology.
“There will be seed companies quite interested in this, I’m sure,” said Bradford, a distinguished professor emeritus in the department and founder of the UC Davis Seed Biotechnology Center. “This is a totally new target to focus on for increasing seed size.”
While the discovery has potentially profound possibilities for expanding the world’s food supply, the backstory is equally compelling. It’s a tale of the value of happenstance encounters at professional gatherings, the ideas that spark when scientists reach across borders, and how science sometimes takes decades to develop knowledge, evolve technology and raise up people who can create something new inspired by earlier advances.
In the case of the callose gateway, that journey has taken 35 years and a sprinkle of serendipity.
Chance meeting in France leads to collaboration
In 2023, Bradford traveled to Paris for a meeting of the International Society for Seed Science. He walked into a session already under way and immediately realized the speaker was talking about a role for callose in controlling how nutrients from the mother plant get into the developing seeds. The speaker, Ryushiro Kasahara of Nagoya University in Japan, was describing how seed size in the weedy cress, Arabidopsis thaliana, can be modified by a gene that codes for an enzyme known as AtBG_ppap; that enzyme can break down callose.
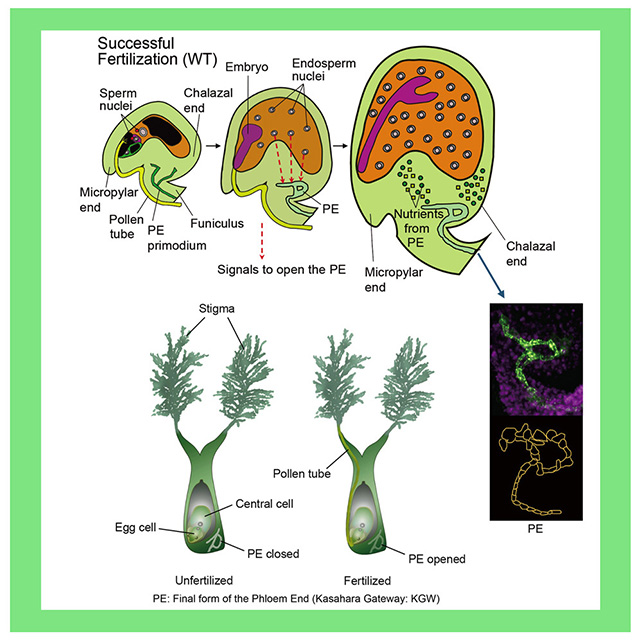
Here’s how it works: A tubelike structure called the phloem delivers nutrients throughout the plant. It also extends to each of the plant’s embryonic seeds; the phloem’s job is to deliver sugars and amino acids the seed needs to grow. At the seed end of the phloem, a blob of callose forms a “gate” blocking the flow until the seed is fertilized. It’s a way of conserving energy: No nutrients get delivered until pollen fertilizes the seed, starting embryo development. When that happens, the AtBG_ppap enzyme breaks down the callose, essentially opening the phloem’s “gate,” so nutrients can flow into the baby seed and support its growth.
Kasahara and team found that, by overexpressing that gene, the callose “gate” opens up even more, letting in more nutrients and growing bigger seeds.

This rang a big bell for Bradford: Back in the late 1980s, he and his team were in the then-Department of Vegetable Crops at UC Davis, and they also were exploring the role of callose in seeds. “We were working on melon seed production,” Bradford recalled. “There’s a disorder that makes the seeds split open.” It’s called “fish mouth,” and it’s a big problem for seed producers. “We were trying to figure out what caused it and how to prevent it.”
Bradford’s team had been looking at the tissues and membranes that form around the developing seed. “We found callose in the cell walls of the outer layer of a particular cell type, called the perisperm,” Bradford recalled. “It can let water go in and out, but it doesn’t let any dissolved solids in or out.” That including nutrients such as sugar, nitrogen, protein and other substances contained inside the seed. However, the team found that, if they exposed the tissues surrounding the embryo to a callose-degrading enzyme – in this case, b-1,3-glucanase – it broke down the callose and allowed the solutes inside to escape, Bradford said. The team eventually published two papers on their work, in 1990 and 1998.
At the same time, Bradford’s team also was conducting research on how water is related to seed development. He hypothesized that the region where the phloem dumps nutrients into the developing seed likely needs some type of cell wall barrier to prevent those nutrients from diffusing away before the embryo can absorb them. The research on melon seeds suggested that callose might serve this role.
An idea takes decades to germinate
More than 30 years later, Bradford saw a key connection between that earlier work and what he was hearing now from Kasahara: the role of callose in controlling the flow of nutrients moving in and out of the seed.
Excited about the connection, Bradford followed up with Kasahara. He learned that the Japanese team had found that opening the callose gateway by overexpressing the AtBG_ppap gene made Arabidopsis seeds 17% larger than usual. Eventually, the team went on to see if there would be a similar enlargement in rice seeds. There was, with rice grains growing 9% bigger when the callose gateway opened wider.
The two scientists developed a rapport that contributed to Kasahara’s understanding of seed formation, Kasahara said. “Dr. Bradford kindly sent me his previous works and suggested an approach I could take in writing the paper. He especially contributed the part about nutrition and water flow,” Kasahara wrote.
Kasahara eventually asked Bradford for help editing the paper summarizing the team’s results. With that assistance, the paper grabbed the attention of journal editors. Kasahara had been trying for seven years, he added.
After getting published in Current Biology, a sister journal to the prestigious Nature, the study drew the attention of the rival journal Science. In an essay published in the April 18 edition, writer Erik Stokstad called the discovery “one with potentially major payoffs for agriculture.”
That’s because the Kasahara team’s discovery points toward a new pathway for channeling a plant’s energy ever more effectively into producing harvestable seeds, Bradford said.
Lingering questions point to possibilities ahead
Bradford still has questions that swirl with callose, pressure gradients and the physics of water flowing through plants. Pondering the Kasahara team’s findings, Bradford noted that, after the seed is fertilized and the callose gate opens, the concentrated nutrient solution flows from the phloem and around the embryonic seed. Much of that water, sans nutrients, flows back out again through the open gateway and back into the plant, Bradford said.
But what keeps the nutrients from flowing out, too? Kasahara’s team found that the callose blob at the seed end of the phloem doesn’t completely disappear. A callose ring remains in place.
It is possible that the callose ring somehow allows water to leave the region of the developing seed, while blocking the exit of dissolved nutrients, Bradford speculated.

Bradford remains in contact with Kasahara and is encouraging the younger scientist to keep exploring the secrets of callose. Those research papers from the 1990s, which Kasahara had not seen before meeting Bradford, are offering ideas. “Now I am thinking about the future strategy for my research, and I would love to collaborate with him,” Kasahara wrote.
The discovery also illustrates a deeper concept underlying plant biology, Bradford said.
“Plants are totally about obtaining and controlling water and managing the flow of water versus the movement of solutes within their tissues,” Bradford mused. “Biology is all about forming separate spaces – and that’s what cells are – to have some control over the internal environment where metabolism and development happen. If we can figure out how callose helps plants to manage the movements of both water and dissolved nutrients in plants, it could help us develop crops that are more efficient in obtaining water and nutrients from the soil as well as growing bigger seeds.”
Read more about callose
Read Kasahara’s paper in Current Biology, “Fertilization-dependent phloem end gate regulates seed size.” Bradford is among the authors.
Earlier papers on callose by Bradford and team:
- Water Relations of Seed Development and Germination in Muskmelon (Cucumis melo L.): IV. Characteristics of the Perisperm during Seed Development. Gregory E. Welbaum , Kent J. Bradford. 1990
- Water Stress and the Water Relations of Seed Development: A Critical Review. Bradford, Kent J. Crop Science, January 1994.
- Callose Deposition Is Responsible for Apoplastic Semipermeability of the Endosperm Envelope of Muskmelon Seeds. Kyu-Ock Yim , Kent J. Bradford. Plant Physiology, Volume 118, Issue 1, September 1998
More research about the role of callose in cell wall construction from the lab of Georgia Drakakaki, in the UC Davis Department of Plant Sciences, is described in these articles:
- Drakakaki Lab animation demystifies how plant cells divide
- Sinclair shows three phases of building a cell wall using 4D imaging
In a recent paper, Maeli Melotto, also in the Department of Plant Sciences, found callose deposition related to the persistence of human pathogens in lettuce leaves was different in different varieties of lettuce.
"It is interesting that this often-forgotten polysaccharide is so critical in so many aspects, from cell division to seed development to defense," Drakakaki remarked.

Media Resources
- Trina Kleist, UC Davis Department of Plant Sciences, tkleist@ucdavis.edu, (530) 754-6148 or (530) 601-6846
